مقالات - Cascade rinsing
The importance of employing standard and efficient equipment in improving the quality and efficiency of the electroplating process.
Cascade rinsing
Reza Mehtar Ghorehdaghi (MSc in Corrosion and Materials Protection)
Peyman Samadi (MSc in Materials Engineering and Metallurgy)
Abstract:
Regardless of the type of electroplating process, a common feature of most standard electroplating lines worldwide is the presence of multiple rinsing tanks. Since the effluents from these tanks constitute a significant volume of wastewater, it is necessary to minimize water consumption during the rinsing processes. By doing so, not only water consumption can be reduced, but also the volume of wastewater and associated costs can be minimized. Nowadays, the use of continuous multi-stage rinsing systems, aiming to minimize water consumption during washing processes, is a legal requirement in advanced countries. By employing this method, the highest washing efficiency is achieved alongside the lowest water consumption. While a brief mention of this method was made in the previous article, due to its importance, this article focuses on it in a more thematic manner.
Introduction:
What is the reason behind the importance of washing, and why is this seemingly simple process significant? Perhaps a mention of the fact that in modern industrialized countries, in a standard nickel-chrome plating line, out of every four tanks present in the line, three are allocated to the washing process, is sufficient to highlight the importance of this stage. In fact, in standard lines, the washing stage must be employed after each main stage to achieve the following objectives:
1. Halting chemical reactions of the solution with the workpiece.
2. Preventing contamination of subsequent solutions due to the transfer of solutions from previous stages into them.
3. Preventing changes in the surface conditions of the workpiece and the formation of spots due to the remaining solution on it.
Proper washing with minimal human labor and water consumption has been an ideal goal that is often challenging to achieve. However, due to the economic and environmental implications associated with it, it has always been considered as the ultimate desirable objective. Reducing water consumption not only reduces the costs associated with water supply but also leads to a reduction in the volume of wastewater generated from the line, making its economic justification viable. With this approach, the environmental and economic implications of reducing water consumption become apparent.
Unfortunately, due to the cheapness of water in the country and the lack of strict monitoring and management of electroplating units, most operators in this field discharge an unacceptable volume of wastewater into the sewage systems without considering the implications of their activities. In advanced countries, electroplating units are obliged to take measures to meet their water needs by adopting efficient management methods. For example, if a unit requires fifty cubic meters of water per day, they are only allowed to use twenty percent of their needs from the municipal water system, and the rest must be provided through the recovery of workshop wastewater. With this approach, owners of such facilities not only understand the necessity of protecting their water resources but also strive to minimize water consumption in various stages of electroplating lines.
In fact, players in the electroplating industry worldwide have redefined the concept of “effort required for plating a part,” where in the past, this phrase was more synonymous with the total number of hours worked per person. However, with the increase in chemical costs and the environmental and water scarcity crises we face today, industry players have also taken into account the implications of these issues in redefining the above phrase. In other words, they have emphasized the optimal use of resources throughout the electroplating process. As mentioned, one of the stages that plays a significant role in determining the amount of water consumed by an electroplating line is the rinsing stage, where efficient methods can significantly reduce the required water. In this article, we will first review the indicators related to the rinsing stage and then discuss the impact of employing multi-stage rinsing systems.
The necessity of washing and dilution ratio:
In general, it is necessary for parts to be rinsed between different rinsing stages. If a part moves from an alkaline washing stage (such as degreasing) to an acidic rinsing stage without rinsing, it will become contaminated and salt deposits will form on its surface, increasing the risk of coating defects. Subsequently, if rinsing is not performed after this stage, the main plating solution will become contaminated. On the other hand, if rinsing is carried out in contaminated water, it can lead to the deactivation of part surfaces or the occurrence of side reactions and the formation of deposits inside the rinsing tanks. Therefore, dilution and refreshing of rinse tanks are vital issues.
Proper and standard washing, by immersing the part in a tank containing clean and flowing water, leads to the dilution of residual solution on the parts. In this regard, there is a parameter called the dilution ratio, which is calculated using the following relationship. In some cases, this parameter is also referred to as the washing factor.
The important issue is the numerical values defined for each of the stages. In other words, the permissible values for each element in post-primary rinsing stages will effectively determine the required water flow rate. It is worth mentioning that the permissible limit for pollutants in electroplating wastewater in European countries is very strict. For example, the permissible limits for elements such as nickel, copper, and zinc are 1 ppm, and for chromium, it is 0.1 ppm. By using the relationship related to the “dilution factor,” the water consumption rate can be determined, and if the permissible concentration of pollutants in the final wastewater is known, the required water volume to achieve the desired concentration can be estimated. To better clarify this equation, an example is provided below.
Example: Nickel-plated parts are washed in a rinsing tank with a flowing water stream. The drag-out rate of wastewater into the rinsing tank is estimated to be 4 liters per hour. If we want to dilute this wastewater with a ratio of 2000, how much water per hour will we need? It is assumed that the concentration of nickel metal in the plating bath is 75 grams per liter.
If we assume that washing is carried out only in a single rinsing tank (overflow-type), according to the provided equation, we will have:
Dilution ratio = (4 + x) / 4 = 2000
Considering the inflow rate of wastewater (drag in) into the rinsing tank and assuming that dilution is performed with a ratio of 2000, the required water rate will be approximately 8000 liters per hour. It should be noted that a dilution ratio of 2000 is approved by reputable companies worldwide and is a logical, standard, and common figure.
In this context, there is a parameter called the dilution constant, denoted by the letter k, which is the inverse of the dilution ratio:
Using this parameter, the concentration of the desired element in the rinsing tank can be calculated. By multiplying the initial concentration (C0) by the dilution constant, we can obtain the concentration of the desired element (C) in the rinsing tank. Considering the concentration of nickel metal in the original solution (75 g/l, equivalent to 75000 ppm), we will have:
9.375=75000×(8000/1)ppm
In fact, in this example, by knowing the water flow rate, we were able to estimate the dilution of wastewater in the final rinse. Now, if we assume that the permissible limit of nickel concentration in the final rinse is approximately 10 ppm, the required water flow rate for dilution using only one overflow rinsing stage will be about 8000 liters per hour, which is unrealistic and economically unjustifiable.
The main objective of using multi-stage rinsing is water conservation, whereby dilution of wastewater is carried out in several consecutive stages instead of a single stage, resulting in encountering progressively diluted wastewater at each stage. Further explanation about this method will be provided later.
شستشوی چند مرحله ای:
Multi-stage washing has been shown to significantly save water consumption by increasing the number of washing stages from one to two. As mentioned, the basis of this phenomenon is the increase in the number of dilution stages and consequently the reduction in pollutant concentrations at each stage. To clarify the nature of this method, schematic diagrams illustrating the effect of increasing the number of rinsing stages on the water requirement rate are shown in Figures 1 to 3. It can be observed that with an increase in the number of rinsing stages, water consumption decreases significantly. This is because with an increase in dilution stages (overflow rinsing), the need for fresh water at each stage decreases compared to the scenario where only one washing stage is used.
In the case of having only one rinsing, the requirement for fresh water per volume of wastewater will be 999 in every hour. However, with an increase in the number of rinsing stages to two, the need reduces to approximately 31 volumes of water per each of the washes, totaling 62 volumes per hour for both. Ultimately, for a system with three rinsing tanks, this number decreases to 27 volumes.
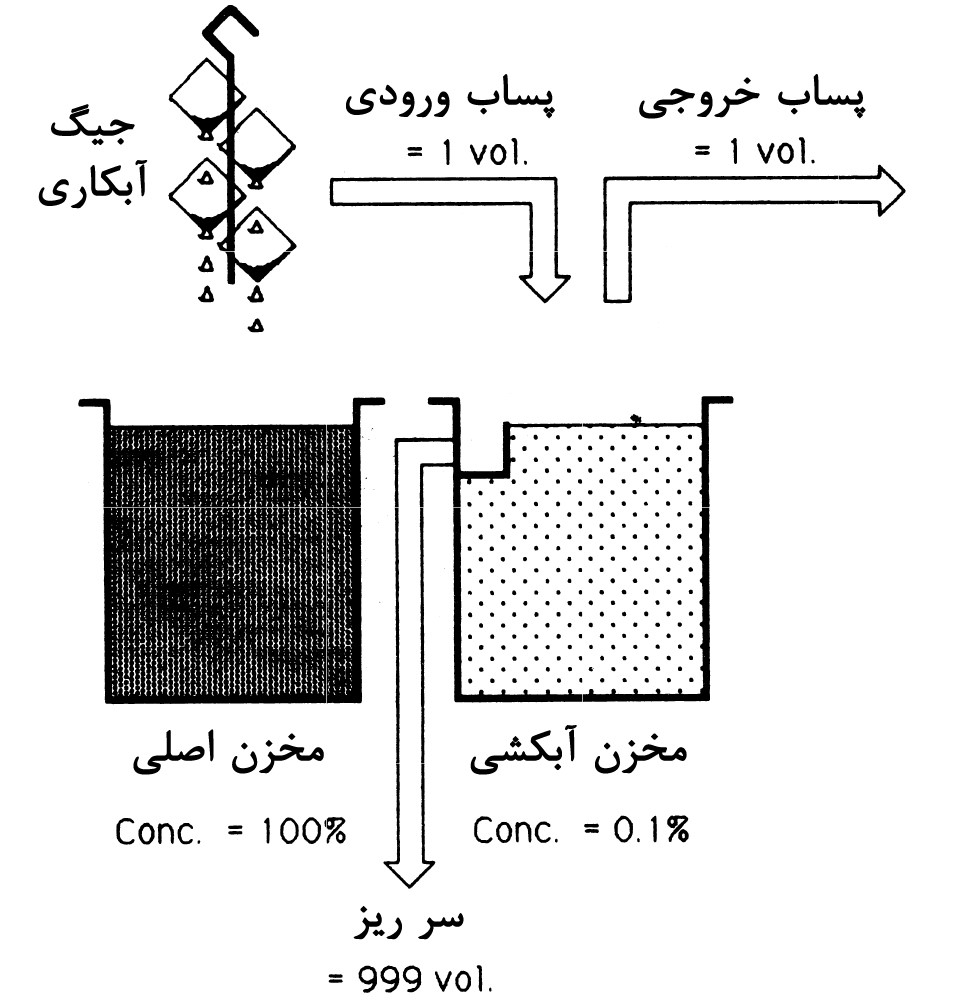
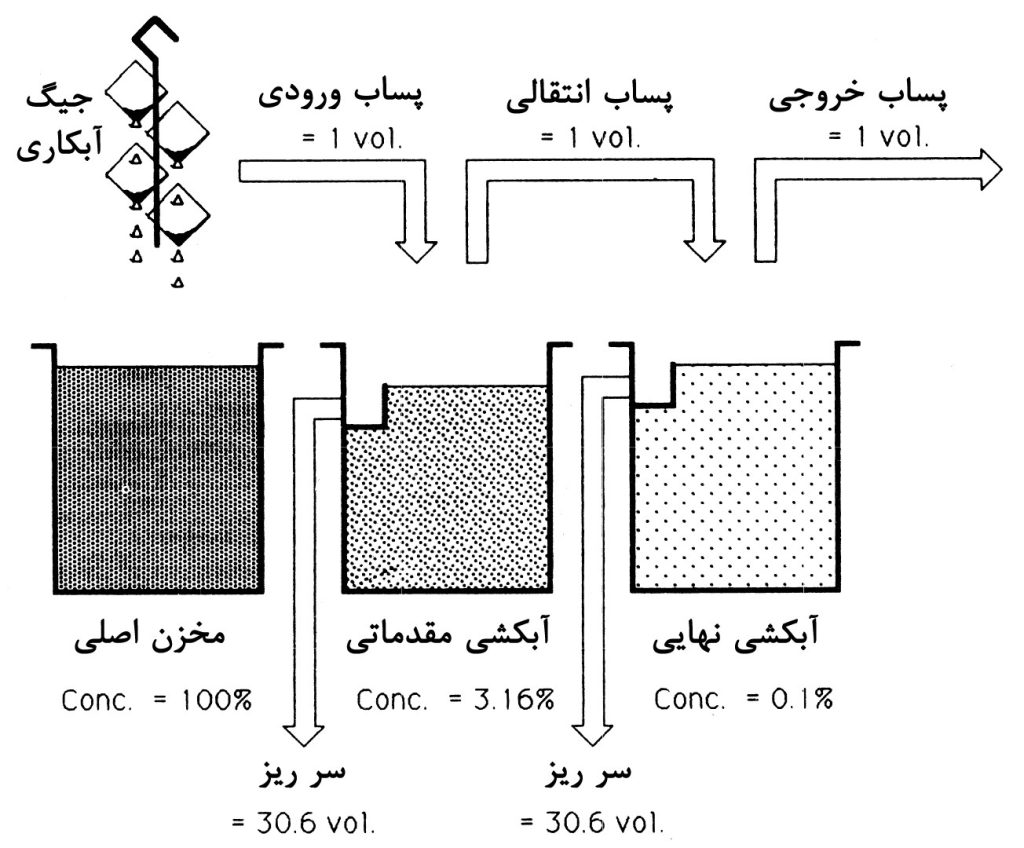
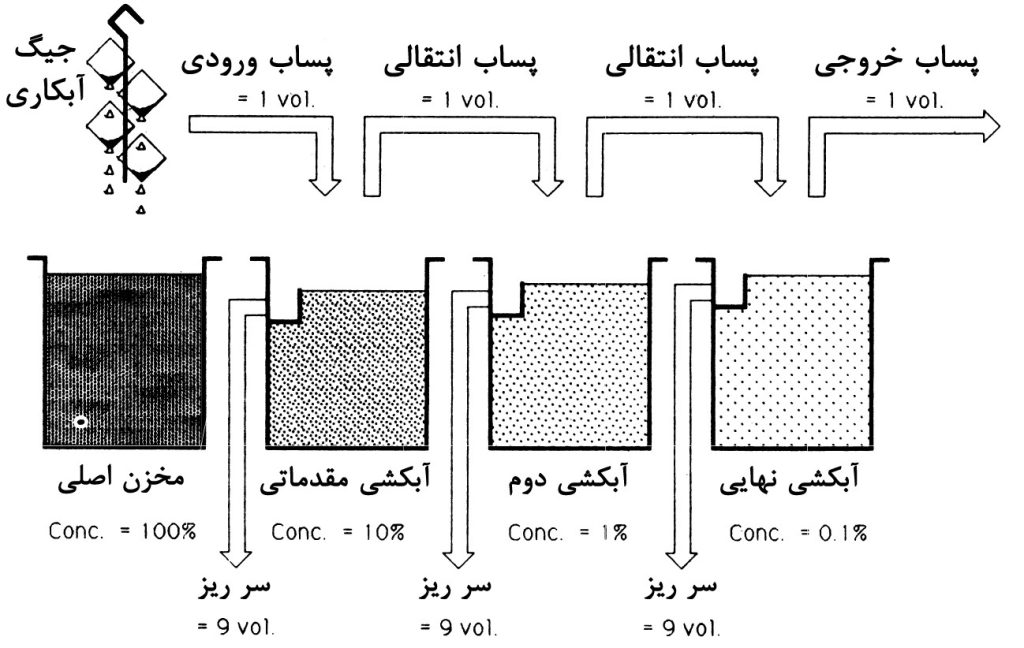
As observed, this method, by employing separate washing tanks and increasing dilution stages, has managed to reduce water consumption. Now, if we design the washing system in a way that the inlet water flow passes through all the tanks, water consumption will further decrease. Next, we will introduce the continuous multi-stage washing process.
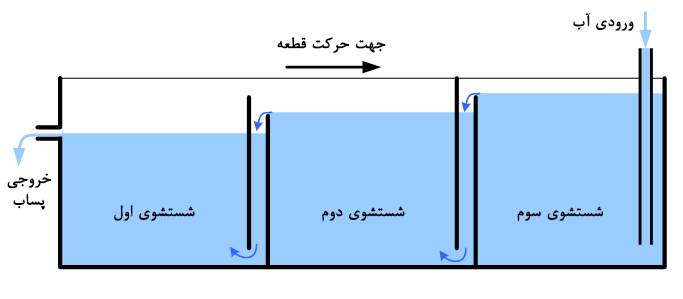
Cascade rinsing:
As mentioned, in an ideal washing process, parts should be cleaned with the least amount of water possible. Nowadays, one of the most effective methods in surface treatment lines worldwide is the use of cascade rinsing. This system is similar to the previous method, with the difference that the overflow from each tank enters the previous tank, thus requiring only one inlet and one outlet. Figure 4 illustrates an example of this system.
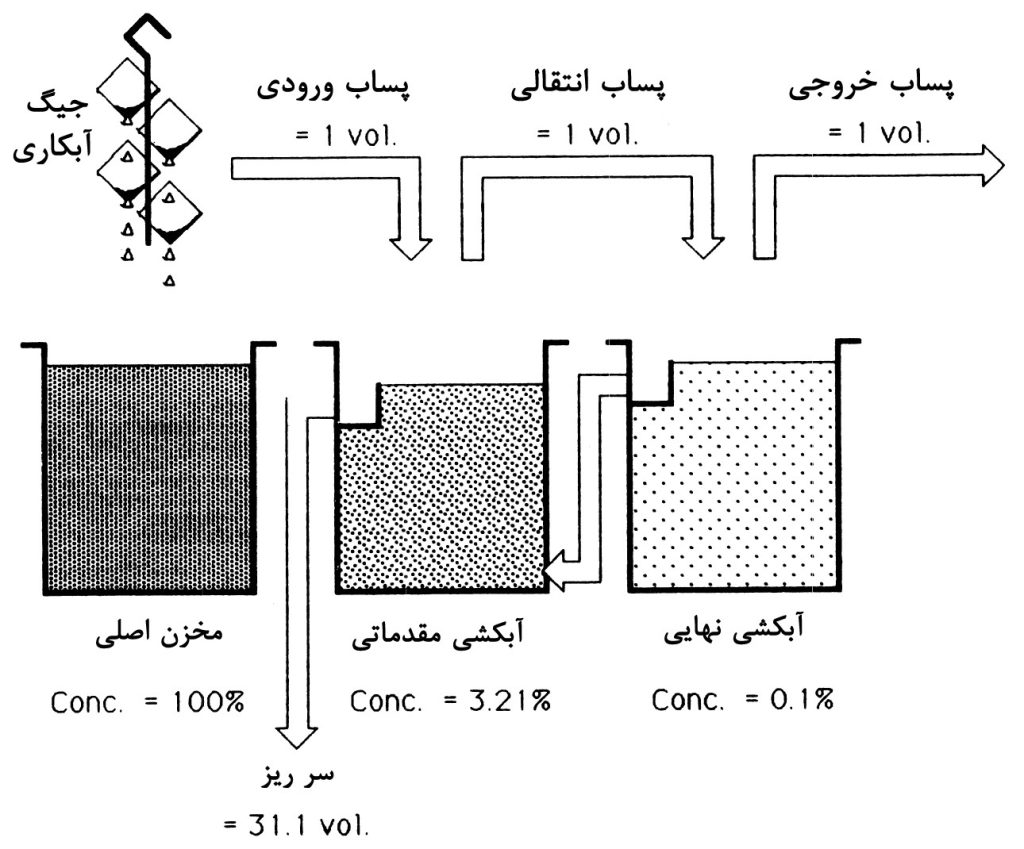
In this system, by using multiple continuous stages, efforts are made to reduce the concentration of metal elements and other pollutants such as cyanide in the wastewater output to the lowest possible level while using the least amount of water. The water consumption rate in this system is also determined by the permissible dilution ratio for each element and the type of surface treatment process (e.g., barrel or rack). For barrel surface treatment, a higher water flow rate is required, which is influenced by factors such as barrel size, hole size, and part geometry, affecting the drag-out rate from the main solution. The minimum reported water consumption rate for barrel surface treatment is typically 50 to 100 liters per hour. For rack surface treatment, it is recommended to use spray washing in the final stage. As mentioned, the dilution ratio varies. Nowadays, in Europe, it is recommended that the dilution ratio be during pre-treatment processes, meaning that for every unit volume of incoming wastewater, 999 units of water should be added to achieve this dilution ratio. Similarly, the recommended dilution ratio after the surface treatment process and after any finishing process such as passivation is different and is less commonly met due to its strictness.
It should be noted that the amount of surface treatment performed per hour is also one of the effective indicators in determining the required water flow rate. Once again, it is important to emphasize the importance of environmental protection and the necessity of water conservation. Measures such as slow removal of parts from the solutions, increasing dwell time, using inclined plates in tank junctions, and designing suitable fixtures are recommended. Below, numerical comparisons are provided to better illustrate the subject. If the drag-out rate from a plating tank, such as nickel, is approximately 3.7 liters per hour and we want to reduce the concentration of elements in the final wash to 15 ppm for chromium and 38 ppm for other metals like nickel and zinc, the table below presents the numerical values for the required water flow rate at different initial concentrations and types of single to multi-stage washing. These numbers are derived using the governing equations.
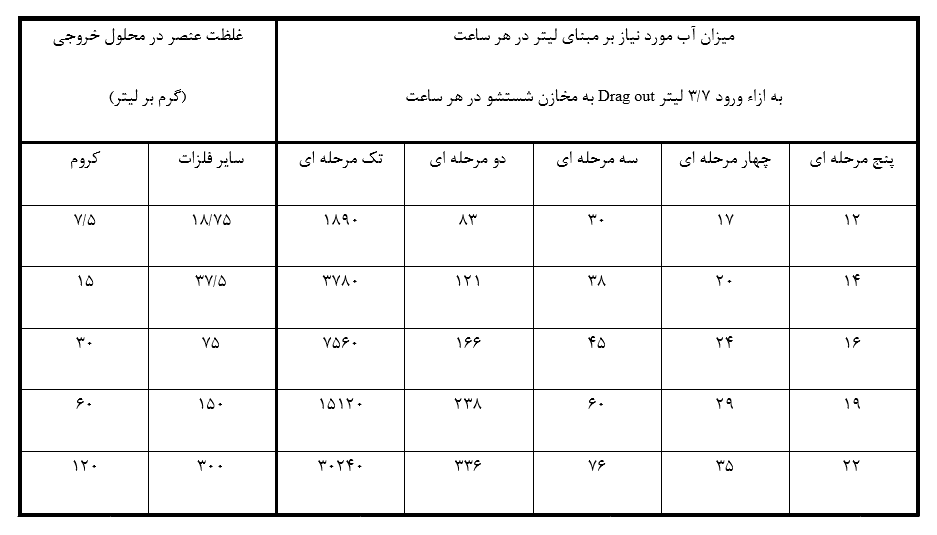
As observed, if the chromium concentration in the drag-out wastewater is 120 g/l, and if we only use a single-stage cascade rinsing, the need for water will be more than thirty thousand liters per hour. However, by employing a three-stage rinsing system, this number will decrease to less than eighty liters per hour, which is a very significant difference. Figure 5 illustrates an example of a three-stage cascade rinsing tank. Additionally, with an increase in the number of rinsing stages, water consumption will further decrease, usually determined by the governing relationships between the initial concentration and the permissible concentration of the pollutant in the rinsing tanks.
Finally, it is worth noting that the more stages in the cascade rinsing process, the lower the water consumption will be, and the concentration of pollutants in the wastewater output will decrease with higher efficiency. Simultaneously, the quality of rinsing increases due to the adherence to the dilution factor. It is also recommended to use an air agitation system on the rinsing tanks to prevent pre-used water from entering the overflow, thus preventing waste.
Here are some key points to consider when employing cascade rinsing:
1- As the impurity concentration in the solution increases, the required water flow rate will increase, regardless of the number of rinsing stages. It is worth noting that the concentration of wastewater has a direct relationship with the volume of drag-out.
2- If only single-stage rinsing is utilized, the required water flow rate for rinsing will increase proportionally based on the impurity concentration in the wastewater.
3- The required water flow rate significantly decreases with the addition of just one rinsing stage.
4- The most significant changes in water consumption occur between single-stage and two-stage rinsing, while the least changes in water flow rate occur when increasing the number of rinsing stages from four to five.
5- Reducing the volume of drag-out and minimizing its transfer to the rinsing tanks takes precedence over reducing the wastewater concentration by employing cascade rinsing. In other words, effective methods should be employed to prevent the transfer of solution from primary tanks like nickel and chromium tanks to the rinsing tanks as much as possible.
Summary:
Unfortunately, a significant portion of stakeholders in the field of water treatment, despite being aware of the reckless water consumption by their respective units, lack any practical plans to reduce water usage. They merely seek ways to secure water, such as drilling unauthorized wells, purchasing low-quality water, etc. Perhaps the main reason for this behavior could be attributed to their clandestine activities and lack of concern for the consequences of their actions on the environment, on one hand, and the affordability of water on the other hand. This lack of attention leads to various problems, including the generation of a large volume of wastewater, which usually directly pours into sewage systems due to the absence of wastewater neutralization systems. Meanwhile, the use of cascade rinsing systems not only significantly reduces water consumption but also enhances the efficiency of water treatment and reduces the volume of wastewater from the production line. In conclusion, if we consider the phenomenon of water conservation not merely from a material perspective but also from an ethical standpoint, assessing our actions in terms of resource wastage, we will undoubtedly reconsider our approach to water consumption fundamentally.









